Pluvicto
Revolutionary Radiopharmaceutical Treatment Provides Hope for Prostate Cancer Patients
Arizona Oncology is proud to offer a leading-edge treatment combining the use of PET scans and a new therapy targeting prostate-specific membrane antigen (PSMA)-positive cancer cells in men with prostate cancer that has spread and become resistant to other forms of treatment.
This new therapy, which was approved by the US Food and Drug Administration in March 2022, uses a tracing agent (ligand) that attaches to PSMA, a protein on the surface of most prostate cancer cells. If this tracer binds to the cancer cells, they light up on a PET/CT scan, and patients are then eligible for a drug called Pluvicto™, which is injected into the bloodstream to deliver targeted radiation to cancer cells throughout the body that express PSMA. The treatment targets bone, nodal, and visceral metastases.
Pluvicto™ is the First and Only Treatment That Targets PSMA+ Cancer Cells Wherever They Are in the Body
“Pluvicto provides us with an important new and unique treatment technique for dealing with the difficult problem of metastatic prostate cancer that has become resistant to the effect of androgen (male hormone) depriving medication.”
— DR. DAVIS MD , RADIATION ONCOLOGIST
Who Qualifies?
Adults with prostate-specific membrane antigen-positive metastatic castration-resistant prostate cancer (PSMA-positive mCRPC) that:
- Has spread to other parts of the body (metastatic)
- Has already been treated with other anticancer treatments
How Does It Work?
Pluvicto™ is a targeted therapy that delivers radiation treatment directly to PSMA+ cells, including tumors. It is given via IV injection or infusion approximately every 6 weeks for up to 6 treatments, depending on how the patient responds.
What Kind of Results Has Pluvicto™ Produced?
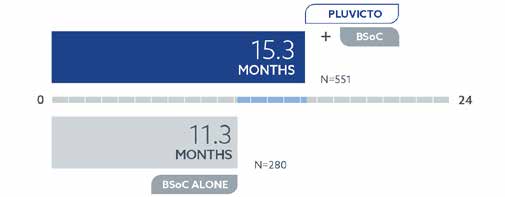
Pluvicto™ Helped Men Live Longer:
Men with PSMA+ mCRPC who received Pluvicto™ plus the best standard of care lived a median of 4 months longer: 15.3 months vs. 11.3 months with BSoC (best standard of care) alone.*
*The Pluvicto™ clinical study measured overall survival (OS). This is the total time men with metastatic prostate cancer were alive from the start of treatment. Median OS is the length of time half of the men were still alive. In a study of 831 men with PSMA+ metastatic prostate cancer, 551 were treated with Pluvicto™ once every 6 weeks (up to 6 treatments) plus BSoC as determined by their doctor. Another 280 were treated with BSoC alone.
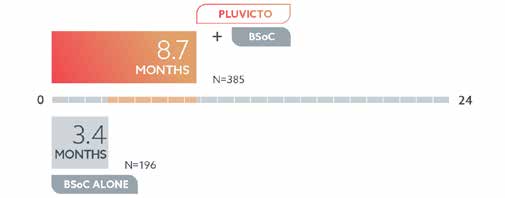
Time Without Progression:
Men treated with Pluvicto™ plus BSoC lived longer without their cancer growing or spreading—a median of 8.7 months compared with 3.4 months when on BSoC only.**
**The Pluvicto™ clinical study measured radiographic progression-free survival (rPFS). This is the length of time men in the study lived with PSMA+ mCRPC without it spreading or getting worse. Median rPFS is the length of time when half of the men were still alive without their cancer spreading or getting worse.