
May 6, 2020 | Southern Arizona
Metastatic breast cancer, which may also be referred to as Stage IV breast cancer, indicates that cancer has spread from the breast tissue and the nearby lymph nodes to other organs in the body, most commonly the bones, lungs, liver or brain. Any type of breast cancer (estrogen-positive, HER2-positive, etc.) can metastasize (spread) to other areas of the body.
When a tumor is found outside of the breast, it’s made up of breast cancer cells. For example, if you have a tumor in the lungs that is metastasized breast cancer, it contains breast cancer cells, not lung cancer cells. These cells may no longer react to the treatments given in the past, meaning that new cancer therapies may be necessary.
The sooner you can detect cancer that has spread to other areas of the body, the easier it will be to contain and treat. That means it’s important to know some of the signs of metastatic breast cancer.
What Are the Signs of Metastatic Breast Cancer?
You will usually show some of these signs before receiving confirmation that the breast cancer has spread. However, you may not associate these symptoms with metastatic breast cancer when only one or two appear. Be sure to talk to your oncologist if you notice any of the following:
- Consistent back, joint, or bone pain
- Confusion
- Severe headache
- Difficulties urinating. This can include incontinence or not being able to urinate at all. This happens when the cancer pinches the nerves in your back.
- Numbness or weakness anywhere in your body
- Difficulty breathing
- Chest pain
- Bloating, pain, or tenderness in the abdominal region
- A constant dry cough
- Vision issues, such as blurry vision, loss of vision, or double vision
- Shortness of breath
- Chest pain
- Loss of appetite
- Balance issues
- Signs of jaundice, such as a yellow tinge to your skin or the whites of your eyes
- Consistent nausea, vomiting, or weight loss
- Seizures
These symptoms can also develop from other medical issues. After talking to your oncologist, he or she may ask for some tests to be run to see if there are breast cancer cells in other areas of the body.
How Does Your Oncologist Determine Metastatic Breast Cancer?
If you’re seeing a different cancer care provider than you did on your last round of treatment, it’s a good idea to bring all of your medical records with you from the previous oncologist. This will help your current cancer specialist understand the type of cancer, its stage, and what was done to treat it. This information can have an impact on what steps are taken next.
After meeting with the oncologist, there are likely some tests to be run to determine what may need to be done next. Here are some common tests for breast cancer detection:
- Blood tests. This can include tumor markers in some patients.
- Whole-body bone scan and this can include X-rays of specific bones
- PET scan
- MRI of the spine or brain
- Biopsy of any suspicious area
- CT scan of the chest, abdomen, pelvis, and/or brain
- Bronchoscopy for patients with a constant cough or trouble breathing
- Spinal tap to remove fluid from around the spinal cord
- Pleural tap checks the fluid between the chest wall and lungs
- X-ray or ultrasound of the abdomen or chest
Your doctor may start out by ordering a couple of tests and based on the results may request further testing. This is so they can get a clear picture of how extensive the cancer has spread throughout the body. This also helps your oncologist come up with the treatment plan they feel will work best for you.
Treatments for Metastatic Breast Cancer
Once you have confirmation of metastatic breast cancer, and your oncologists have the information they need, a treatment plan can be created. In some cases there is a combination of treatments required. One or more of the following may be included in your treatment plan for metastatic breast cancer:
- Hormone therapies: The most common approach and treatment for metastatic estrogen or progesterone receptor-positive breast cancer is hormone therapy. You may have already used one or more of these in your previous treatments. Based on your history, the oncologist may recommend a new hormone therapy. The most common hormone therapies are:
- Tamoxifen: This treatment blocks estrogen in both pre and postmenopausal women. It’s used primarily in young women who haven’t had hormone therapy before, and it’s designed to contain and shrink the tumors.
- Fulvestrant: After attaching to the estrogen receptors, this drug changes the shape of the receptors and degrades it preventing further tumor growth. This should slow the growth of cancer cells.
- Aromatase inhibitors: These hormones stop the actions of the enzyme aromatase, which is responsible for the conversion of androgens to estrogens. The hope is that this will lower levels of circulating estrogen and stop the growth of cancer cells.
- There are other hormone therapies being introduced for advanced breast cancer. Talk to your oncologist about what is available if you have used these other hormone therapies already.
- Chemotherapy drugs: If you’ve already battled breast cancer, you may find some drugs you’re familiar with while others are used more often for metastatic breast cancer. This is the most common approach for metastatic breast cancer that is :
- Hormone-receptor negative
- Hormone-receptor positive, but doesn’t respond to hormone therapies
- HER2-positive, in combination with a targeted therapy
- Chemotherapy tends to work quicker than hormone therapy, which may also be a consideration for whether it’s used right away.
- Targeted therapies: Most commonly used for HER2 positive breast cancers, this category of drug targets the HER2 protein on the cells which fuels cancer cell development.
- Immunotherapies: These drugs, which help your immune system fight the cancer, are less commonly used for breast cancer, but may be used in combination with other treatments.
- Surgery: Sometimes surgery to remove a tumor that has developed in another area of the body is necessary to avoid further growth. The location of the tumor dictates if surgery is a possibility.
There may also be the possibility of a clinical research trial available through Arizona Oncology for advanced-stage breast cancer. You can discuss your openness to clinical research during your appointment.
If you’ve been previously diagnosed with breast cancer, it’s critical that you attend your regularly scheduled check-ups, even years later. At the first signs of metastatic breast cancer, you need to contact your oncologist.
At Arizona Oncology, we understand your apprehension, and we’re ready to help you at each step of the process.

Apr 24, 2020 | Southern Arizona
Cancer treatments such as chemotherapy and radiation are designed to kill fast-growing cancer cells. These powerful drugs travel throughout the body and may affect healthy, normal fast-growing cells as well. The type of long-term side effects cancer survivors face and the severity differs between individuals. Some may experience minimal long-term effects, while others may experience moderate to severe long-term effects.
There are many different types of cancer and many different types of treatment. This combined with the fact that everyone’s body responds differently makes the possibilities of long-term side effects from cancer treatment endless. Let’s take a look at the most common long-term side effects:
- Side-effects from surgery
- Dental and oral issues
- Heart conditions
- High blood pressure
- Pulmonary conditions
- Side-effects to the endocrine system
- Impaired brain function/chemo brain
- Hair loss
- Secondary cancer
- Decreased bone strength
- Emotional problems
Long-Term Side Effects Related to Surgery
Long-term surgical side effects(1) will depend on the type of cancer you were diagnosed with and the location in the body you had surgery. The following are a few examples of side effects from surgery. Scarring at the surgical site is a common long-term effect as well as chronic pain. Lymphedema, which is painful swelling from fluid build-up is the result of having lymph nodes removed. Survivors who had a limb amputation may experience phantom pain. If your spleen was removed due to Hodgkin’s Lymphoma, you might be at a higher risk for developing infections.
Dental and Oral Issues
Tissues in the mouth are among the fastest-growing tissues in the body. Since cancer treatments attack fast-growing tissues it can cause long-term negative dental and oral side-effects such as:
- Cavities
- Tooth decay
- Dry mouth
- Gum disease
- Loss of taste
It is important to see your dentist if you experience any oral issues after cancer treatment.
Heart Conditions
Chemotherapy and radiation cancer treatment to the chest increases the risk of long-term side effects on the heart. Congestive heart failure, abnormal heart rhythms, and hardened and narrow arteries (coronary artery disease) are the most common side effects, although they may not appear for months or even years after cancer treatment is complete. There is also an increased risk of developing heart issues when taking certain medications as a part of your cancer treatment.
High Blood Pressure
Certain medications used as a part of cancer treatment can cause high blood pressure in patients. These include Bevacizumab, Sorafenib, and Sunitinib.(1) For many patients, blood pressure returns to normal once you are no longer taking the medication. However, for others, it is a long-term side effect that must be managed with medications, diet, exercise, or a combination of treatments to keep your blood pressure under control.
Pulmonary Conditions
Those who received either chemo or radiation to the chest are more likely to experience damage to the lungs. Survivors who received both chemo and radiation are an even higher risk of long-term pulmonary side effects due to cancer treatment. Side-effects include:
- A decrease in pulmonary function
- Difficulty breathing
- Inflammation and thickening of the lining of the lungs
Long-Term Side Effects to the Endocrine System
Cancer treatments may cause long-term side effects to the endocrine system due to the chemotherapy or radiation causing a change in the level of hormones for both men and women. For women, this could mean menopausal symptoms in which your body stops menstruating. For younger women, menstruation may come back at some point after cancer treatments are finished. This is not always the case.
For both men and women, the change in hormones due to treatment can cause infertility, changes in your sex drive, and hot flashes.
Related reading: Fertility for Cancer Survivors
Impaired Brain Function
Chemotherapy and radiation can cause survivors to have long-term trouble with memory, processing information, and staying focused on tasks at hand. Those who received cancer treatment to the head are at a higher risk for developing long-term cognitive issues. This is commonly referred to as chemo brain. While chemo brain is usually worse during treatment, it is known to last after treatment is completed-sometimes for a few years.
Fatigue
Cancer treatment is powerful and can not only leave the patient feeling tired during treatment but for months and years afterward. This is one of the most common long-term side effects experienced by cancer survivors.
Long-Term Bone Issues
Certain cancer treatments may cause thinning of the bones, which is also called osteoporosis. Survivors may also experience joint pain as well. These side-effects can be made worse if the individual is not physically active.
Hair Loss
Hair loss is a common side-effect of cancer treatment. For most people, their hair begins to grow back after treatment. For others, it may not grow back, it may be thinner, and it may come back a completely different color or texture.
Related reading: To Wig or Not to Wig?
Secondary Cancer
Secondary cancers, although not common do happen. It is important to understand that a secondary cancer is not a recurrence of the original type of cancer. It is an altogether different and unrelated cancer that occurs in a different part of the body. For instance, let’s say you successfully completed breast cancer treatment and years down the road are diagnosed with colon cancer. Colon cancer is a secondary cancer.
Certain chemotherapy and radiation treatments for cancer have the potential to increase the chances that an individual will develop a secondary cancer down the road. The younger an individual is when they undergo cancer treatment, the higher the risk of developing a secondary cancer later in life.
Emotional Difficulties
It is very common that cancer survivors experience a range of emotions from relieved and happy to sad, angry, depressed, helpless, and everything in between. From the moment you are diagnosed to when you receive your last treatment and beyond is a roller coaster of emotion for both the survivor and their families. Talking with someone can help survivors work through their emotional difficulties.
Managing Long-Term Side Effects Due to Cancer Treatment
Cancer treatment takes a toll on the mind and body, that is not a secret. It stands to reason that it takes time to begin feeling like yourself again. Arizona Oncology understands that the journey does not end when treatment ends. There are both the physical and emotional side-effects of being faced with and surviving a life-threatening disease. You do not have to go it alone. Our staff offers supportive care to help you manage your long term side effects and adjust to a new normal in life!
Continue reading about Cancer Treatment Tips and Side Effect Management.
Find an oncologist near you.

Feb 6, 2020 | Southern Arizona
If you are preparing for or anticipating having to schedule your first mammogram, you may be wondering what to expect. A mammogram is a non-invasive diagnostic scan essential for early detection of breast cancer. It can be an inexpensive and highly effective method for reducing breast cancer risks. Having regular mammograms can be critical for those with a higher risk level or history of family breast cancer of any age.
Today, we’ll provide a guide to preparing for your first mammogram and outline what you can expect during your first screening.
When Should You Have Your First Mammogram?
Many experts suggest annual mammograms are ideal for women within the 45-54 age range. Annual screenings are often recommended and can significantly reduce the risks of death as a result. In fact, research suggests regular mammograms reduce breast cancer death risks by 14% for women within 50 and 60 years old. Those percentages increase with age; up to 33% for those 60 to 70 years old. Some women may begin mammogram routines earlier in life, depending on individual health conditions, family history of breast abnormalities, or the discovery of a lump. Your health care provider can help you determine when it’s best to begin your annual mammogram screenings.
Scheduling & Choosing a Facility
Your health care team or primary care physician may recommend a facility for your first mammogram. If you don’t have a recommendation to guide you, consider finding an area location that routinely performs mammograms. As you prepare for your first mammogram, you’ll want to choose a site that can perform your annual mammograms in the years to come. Staying with one provider can make it easier to compare results year after year.
Preparing for Paperwork & Forms
You will want to be mindful of your specific insurance carrier information ahead of time. Most insurance providers cover mammogram procedures as a standard, preventative visit. However, it’s best to review your plan to understand what coverages apply to you. You will be asked to present your card at the time of your appointment. It is a common suggestion that you arrive to the appointment a little early, should you have insurance or provider related questions. Being early can also allocate enough time to complete any new patient, or medical history forms the facility may require.
Preparing for the Mammogram
When you are called back, you can expect to be situated in a private room. The technician will provide you with a waist-up gown for you to wear. In preparation before you go, it’s best to make sure you’re not wearing any jewelry or deodorant. It’s also recommended that you not be wearing lotions or skincare products. The types of products can affect the imaging. If you arrive to your appointment having forgotten to abstain from these products, the technician can provide you with a warm cloth or towelette to remove them.
What to Expect During the Imaging Process
You can expect the procedure itself to be relatively brief. The technician will position the machine, and you, in order to take pictures of the breast. Once you are strategically positioned, you’ll be asked to remain completely still and maybe to hold your breath for a few brief seconds during the picture-taking process. You can expect a few different positions for each breast. The technician may leave the room to digitally present the images to the on-staff Radiologist for quality review. If any of the pictures are obscure, the team may have you repositioned for a retake or two. Once they are confident the images are clear, you’ll be free to get dressed and leave.
Are Mammograms Painful?
While you can expect mild discomfort during the mammogram process, it’s relatively painless. Your breast will be positioned on a tray that is adjusted to accommodate your height. A top-level tray will be lowered to hold your breast in place. So, while it can be uncomfortable for a few moments, you won’t encounter any significant pain. As an added tip, try to avoid scheduling your mammogram during the first week of or week prior to starting your period. Because your breasts can be sensitive during this time, the position requirements of a mammogram can be slightly more painful.
Related reading: Breast Cancer Misconceptions
Waiting for Results
Most routine mammogram results will be delivered to you by mail or phone and within ten days. You may be notified sooner, depending on the facility. Upon review of the images, the Radiologist may want additional scans, ultrasounds, or x-rays. Don’t be alarmed if you receive notice to return for another round of images. It may mean there is an unclear area, that one of these other methods of imaging can portray better. Your referring physician can be of assistance during this follow-up and waiting period as well, should you have questions about follow up scan requests. Just know that callbacks can be common, and most prove not to represent breast cancer.
Getting Started on Your Path of Mammogram Routine Screening
It can be scary for some women to consider embarking on the annual mammogram routine. There may always be a subtle concern or anxiety that mammograms will uncover breast cancers. The good news is, screening often results in normal findings. For those instances when abnormalities are present, the early detection alone can be life-saving. If you’re approaching the age of 45, it’s a good idea to add mammograms to your roster of health-promoting and preventative visits. If you’re younger, but have concerns with family history or a recently discovered lump, ask your primary care physician for guidance or referral to an area mammogram facility.
Consider mammograms as a necessary and diagnostic tool that helps better arm women to combat the risk of breast cancer. Images can provide valuable information about your current breast health. They can also serve as a timeline of changing breast health in the years to come. Being able to identify a mass early, or changes to breast tissue can help ensure your best chances of recovery, survival, and health preservation overall.

Jan 7, 2020 | Southern Arizona
Most people assume that symptoms of lung cancer are related strictly to the lungs and complications related to breathing. Sometimes this is accurate. Lung cancer often has symptoms like coughing up blood and mucus, shortness of breath, persistent cough, and chest pain. However, some symptoms seem to have nothing to do with the lungs. While there are many breathing-related symptoms of lung cancer, other surprising symptoms exist. Here are 9 surprising symptoms of lung cancer that don’t involve the lungs.
9 Symptoms That Could Mean Lung Cancer
- Clubbing of the nails
- Hoarse voice or vocal changes
- Weakness or numbness of the arm and shoulder
- Extreme thirst and frequent urination
- Upper body swelling and bruising
- Quitting smoking is suddenly easy
- Horner syndrome
- Stomach issues
- Fatigue and dizziness
While these symptoms don’t seem related to your lungs, there are other things happening in your body when you have lung cancer. Often, lung cancer has few symptoms before it’s in the advanced stages. However, symptoms that occur throughout the body may be misdiagnosed as other illnesses. Learning about the symptoms of lung cancer can lead to early treatment which can save your life. These symptoms can be caused by other conditions, but they can be an indication of lung cancer.
1. Clubbing of the Nails
Changes in your fingers and toes can happen quickly and could signify serious health issues. Clubbing of the nails occurs when the normal shape of your nail bulges or curves outward. The ends of your fingers or toes may appear to bulge and nails may not feel firmly attached. The change is due to excess soft tissue forming under the nail bed, pushing nails away from the skin.
Lung cancer is the most common cause of clubbing. The condition occurs because of reduced oxygen in the bloodstream. Clubbing of the nails generally occurs alongside other symptoms and can be caused by other health conditions like heart defects, chronic lung conditions, certain cancers, and thyroid problems. If you experience clubbed nails, you should visit your doctor. While this symptom doesn’t mean you have lung cancer, your doctor will ask questions about other symptoms to help determine the cause.
2. Hoarse Voice
Changes in your voice that aren’t because of obvious symptoms like a cold or sore throat could be a concern. Hoarseness is common in advanced cases of lung cancer and is sometimes caused by infections and side effects of chemotherapy. However, difficulty producing sounds may also be an early sign of lung cancer. The hoarseness is most often caused by paralysis of the recurrent laryngeal nerve, which passes through the chest close to the left lung.
3. Weakness or Numbness in Arms or Shoulder
Pain in your arm or shoulder without the presence of an injury could be a concern. Pancoast tumors develop in the top of the lung and can compress nerves that lead to your shoulder and arm. Shoulder pain may be sudden and intense or persistent with no apparent cause. Arm pain or weakness is less likely and usually develops over time.
4. Extreme Thirst and Frequent Urination
Thirst is common with the sudden onset of warmer weather, but sudden extreme thirst is a symptom that you should always discuss with your doctor. Some cancers can produce hormone-like substances that cause changes in the body. These substances can cause a group of symptoms referred to as paraneoplastic syndromes. These symptoms can be among the first signs of lung cancer. One such symptom is an increase of calcium in the blood which causes excessive thirst and frequent urination.
5. Upper Body Bruising and Swelling
While swelling and bruising are usually associated with injuries, there are other reasons the condition could occur. The superior vena cava is a large vein that carries blood from the head and arms back to the heart. Tumors pressing on this vein can lead to blood pooling in the veins. This pooling leads to swelling in the upper arms, chest, and neck. Puffy facial features may also be a symptom. The swelling is sometimes accompanied by a blue or red tinted skin.
6. You Suddenly Quit Smoking
It’s no secret that most people who begin smoking have difficulty quitting. However, many patients end up quitting before getting a lung cancer diagnosis. While many patients quit smoking because they’re hoping to alleviate symptoms like coughing or shortness of breath, many patients suddenly find quitting easy. Since patients suddenly have the ability to quit without difficulty, the symptom is likely caused by changes in the body related to cancer. If you would like help quitting smoking, read our blog for helpful tips.
7. Horner Syndrome
Also caused by Pancoast Tumors, Horner Syndrome is caused when the tumors affect the nerves leading to the eye or face. Symptoms include:
- Drooping or weakness of one eyelid
- A smaller pupil
- Reduced or absent sweating on the same side of the face as the affected eye
8. Stomach Issues
It seems like your digestive system shouldn’t really be affected by your lungs at all. However, lung cancer can cause the body to produce hormone-like substances that change the way your body works. One or two out of every 10 cancer patients develop too much calcium in the blood. This can lead to nausea, belly pain, and constipation.
9. Fatigue and Dizziness
Consistent fatigue and occasional dizziness may be caused by different reasons. Anemia (low levels of red blood cells) is common for lung cancer patients which causes chronic fatigue. Additionally, compression of the superior vena cava can affect the brain causing light-headedness or dizziness.
All of these symptoms can have multiple causes. However, it’s important to visit your doctor when you experience persistent medical changes. Lung cancer doesn’t only affect smokers. In fact, it’s the leading cause of cancer deaths among both men and women and often doesn’t exhibit symptoms until it’s in the advanced stages. Early detection and treatment is the best defense against any type of cancer. Watching for the symptoms of lung cancer can save your life.
Arizona Oncology has offices located throughout the state, where you will receive state-of-the art lung cancer treatment in a caring and comfortable environment close to home, work, and family. Our team of physicians and cancer care specialists are ready to help you every step of the way.

Dec 31, 2019 | Southern Arizona
Arizona means living with sunshine year round, even when it’s not super hot outside. That can be dangerous for your skin. Some people believe that the cooler weather in winter decreases their risk of developing skin cancer. The truth is, regardless of the temperature outside, the sun can still cause skin damage. All sun exposure can lead to a higher risk of skin cancer–even in those winter months that aren’t super hot.
Skin Cancer Risks in Winter
Protecting your skin from skin cancer is a year-round job. Why? Because harmful ultraviolet (UV) rays are always being released from the sun. Yes, sunlight is strongest during the summer and surrounding months, but that doesn’t mean you should slack off on skin protection when the sun doesn’t seem as strong in winter.
UV rays still reach your skin through dark cloud coverage, especially at high altitudes. Living in Arizona means that there are opportunities for other year-round activity, such as golf, hiking, mountain biking, snowshoeing, and skiing. This means that the rays are hitting you throughout all four seasons, maintaining that risk of skin cancer and premature aging.
UVA rays can also penetrate glass, so even if you aren’t outdoors, it’s still possible to damage your skin while spending a bright day indoors.
How to Protect Against Skin Cancer in Winter
Skin cancer is the most common cancer in the United States, according to the American Cancer Society (ACS). The good news is that it’s one of the easiest to prevent if you take the proper steps to protect yourself. Some ways you can protect your skin can include:
- Covering up.When it comes to protecting your skin from the sun, clothing is your first line of defense–regardless of the time of year. The face, head, and neck are where the majority of skin cancers occur, so make sure your wardrobe includes UV-blocking sunglasses and a wide-brimmed hat in addition to pants and long sleeves, which can cover larger areas of your skin. The goal is to leave very little skin exposed to the sun as often as possible.
- Using sunscreen.Use a broad-spectrum sunscreen with an SPF (Sun Protection Factor) of 30 or higher whenever you spend time outdoors. Apply generously at least 20 minutes before going outdoors, spreading evenly on all exposed areas. It’s important to reapply to any exposed skin every 90 minutes to two hours. Sunscreen should be reapplied immediately after swimming or sweating and toweling off. Be sure to also protect your lips by wearing lip balm that is SPF 15 or higher. Read more about choosing the right sunscreen for you in our blog on how to read a sunscreen label.
- Scheduling activities around the sun.When possible, try scheduling your activities to where they fall early in the morning or later in the afternoon, when the rays aren’t as strong. UV rays are typically the strongest during the hours of 10 AM and 4 PM.
- Avoid tanning beds. It may be tempting to keep a summer glow by using a tanning bed in the winter. Tanning beds should be avoided at all times because they lamps give out UVA and UVB rays that cause long-term skin damage, which can contribute to skin cancer.
Winter air leads to dehydration that can cause dry skin, which is more prone to damage from UV rays.In addition to the above tips, it’s important to think about hydration, especially when living in the desert. Even during times you may not feel thirsty, it’s important to understand that your body loses water in the same way during colder months as it does during a hot summer through sweating, breathing, and urinating. Furthermore, dehydration can lead to dry skin, which is more prone to damage from UV rays, so it’s vital that you do your best to maintain a good fluid intake. To ensure you’re properly hydrated:
- Drink healthy fluids. Plain or fruit-infused water, healthy fruit and vegetable juices, hot tea (preferably decaf), and even hot water with lemon are good choices. Don’t wait until your thirsty–keep sipping throughout the day.
- Minimize your caffeine and alcohol intake.Alcohol and caffeinated beverages like soda, tea, coffee act as diuretics and can contribute to dehydration.
- Eat foods with high water content. Some foods to keep you hydrated include broccoli, cucumbers, celery, strawberries, and watermelon.
- Check your urine.Light urine means you are likely hydrated enough. Dark urine means you need to increase your fluids.
You can learn more about how you can fight against overexposure to UV light by reading our blog on skin cancer prevention tips.
Remember, extreme UV rays reach Arizona all year long. With that said, there is no reason to fear the sun. Have fun in it, practice sun safety, and encourage others to do it, too!
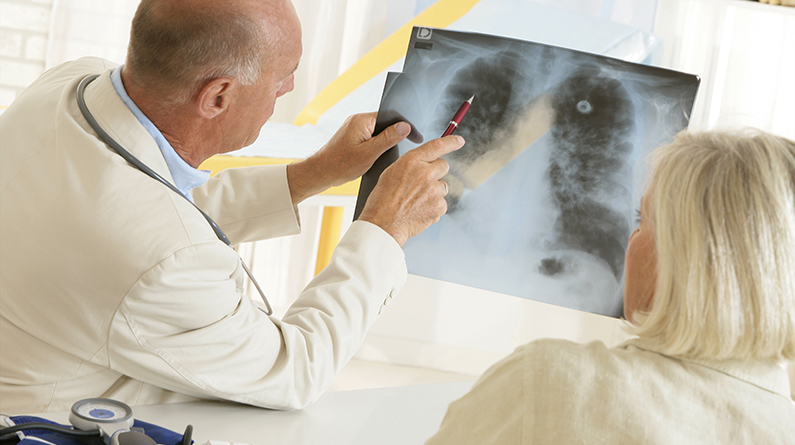
Dec 27, 2019 | Southern Arizona
Lung cancer is the leading cause of cancer deaths in the United States amoung men and women. There are some lifestyle choices you can make to try and reduce your risk of getting lung cancer. Being aware of the symptoms to look for is helpful in catching the disease early and having a better treatment outcome. For people who have a family history of cancer, screening becomes even more important, since that population is at a higher risk of developing the disease. The information below is meant to be used as a guideline. Individuals experiencing any of these symptoms should consult their physician.
Lung Cancer Risk Factors
- Smoking: Smoking is the greatest risk factor for lung cancer. Tobacco smoke causes more than 8 out of 10 lung cancer deaths. Exposure to second-hand smoke increases the risk of developing lung cancer as well.
- Chemical exposure: Some professions are regularly exposed to harmful chemicals which can lead to an increased risk.
- Diseases: Chronic Obstructive Pulmonary Disease, Pulmonary Fibrosis, and Tuberculosis place someone at higher risk for lung cancer.
- Family history: There is an increased risk when immediate family members have had the disease especially at a young age.
Read more about lung cancer risk factors.
Lung Cancer Signs and Symptoms
- A cough that does not go away
- Chest pain, made worse by deep breathing, coughing or laughing
- Hoarseness
- Weight loss and loss of appetite
- Bloody or rust colored sputum
- New onset of wheezing or shortness of breath
- Reoccurring infections such as bronchitis or pneumonia
Read more about lung cancer signs and symptoms.
Lung Cancer Screening
Patients should discuss their health history and individual risk factors with their physician to determine if lung cancer screening is recommended.
Page 17 of 18« First«...10...1415161718»








